
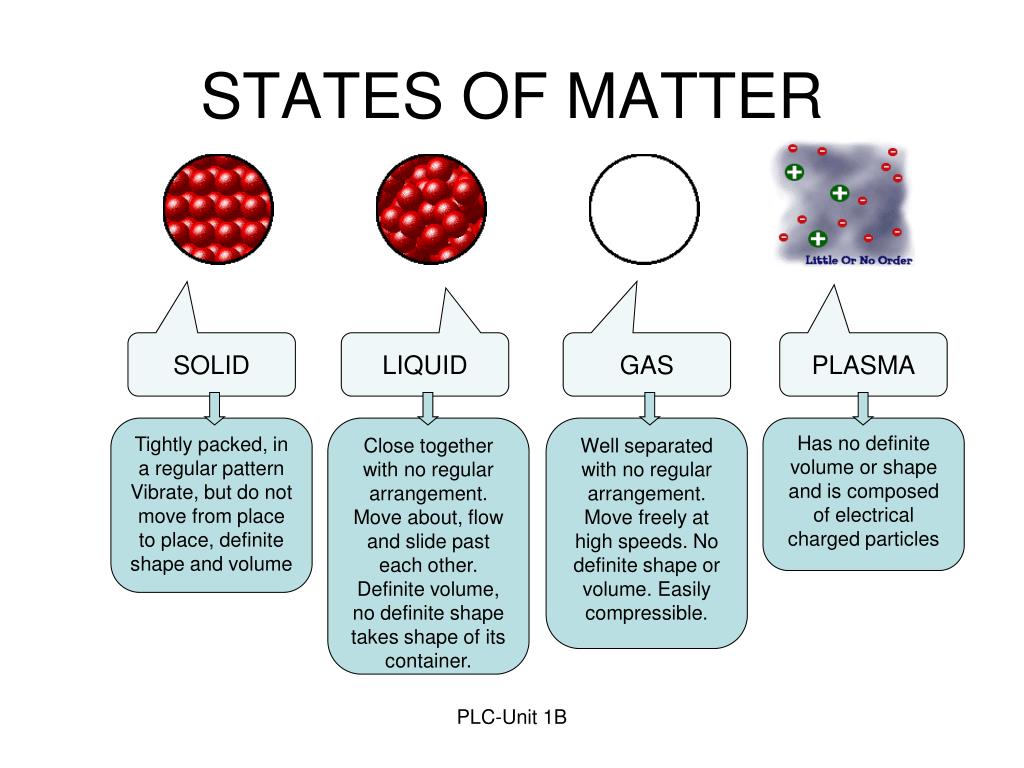
The physical state of a compound is determined by the temperature and the kinetic energy as well as the intermolecular forces in the substance. Ionic compounds never exist as gases since very high temperature is required in overcoming strong electrostatic attraction between the ions. Slowly moving molecules are attracted to each other easily and results in condensed phases: liquid and solids. As the temperature decreased, the molecules of a gas can be condensed to liquids and then to solids. These particles are constantly in motion and possess energy of motion (kinetic energy) that we perceive as temperature. Gases, Liquids, and Solids 7.1 Kinetic Molecular Theory of Matter Kinetic Molecular Theory of Matter The Kinetic Molecular Theory of Matter is a concept that basically states that matter is composed of a very large number of very tiny particles molecules or ions.
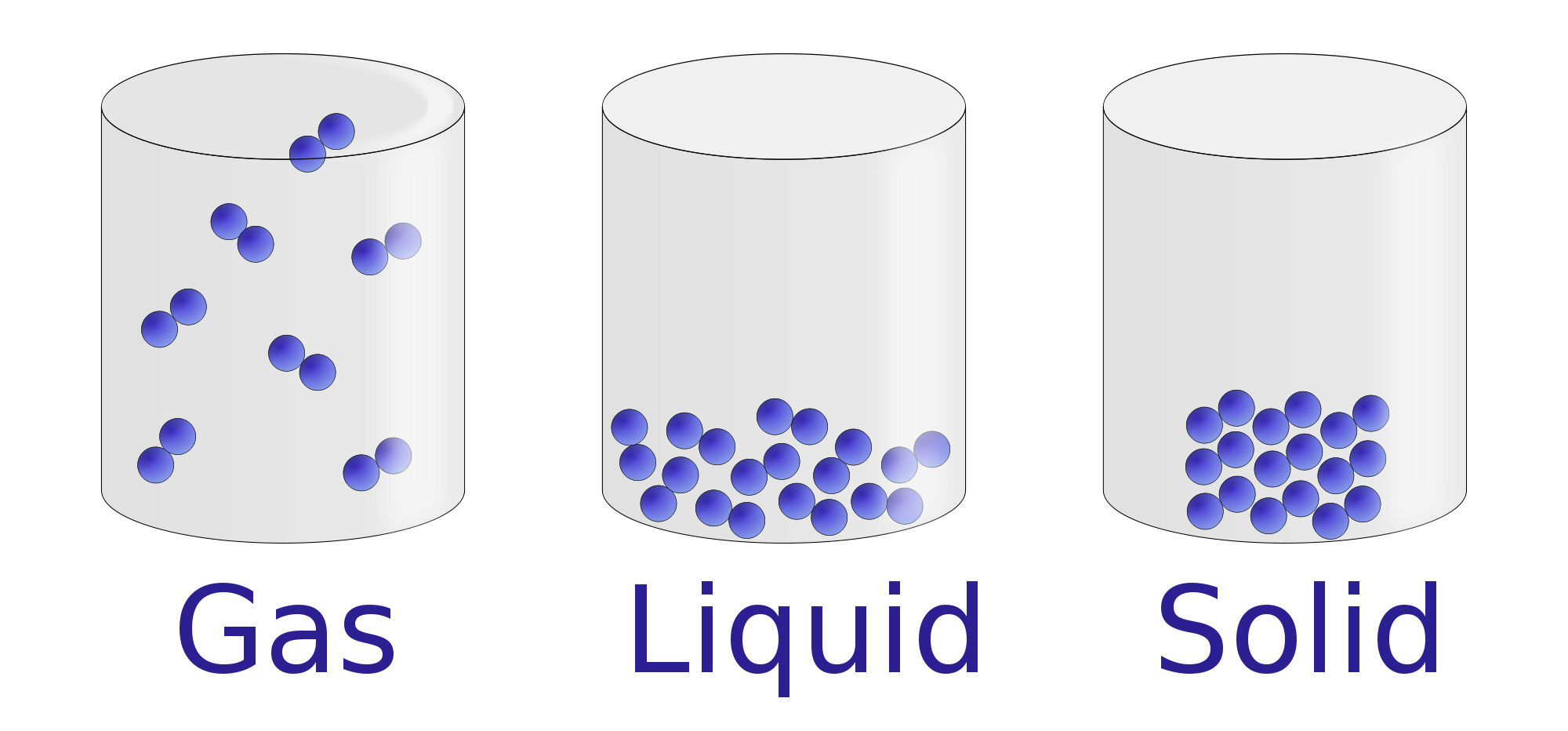
The number of molecules ( N) is very large, to the extent that tracking individual particle behaviors is not possible.Specifically, one implication of this is that their size is extremely small in comparison to the average distance between particles.
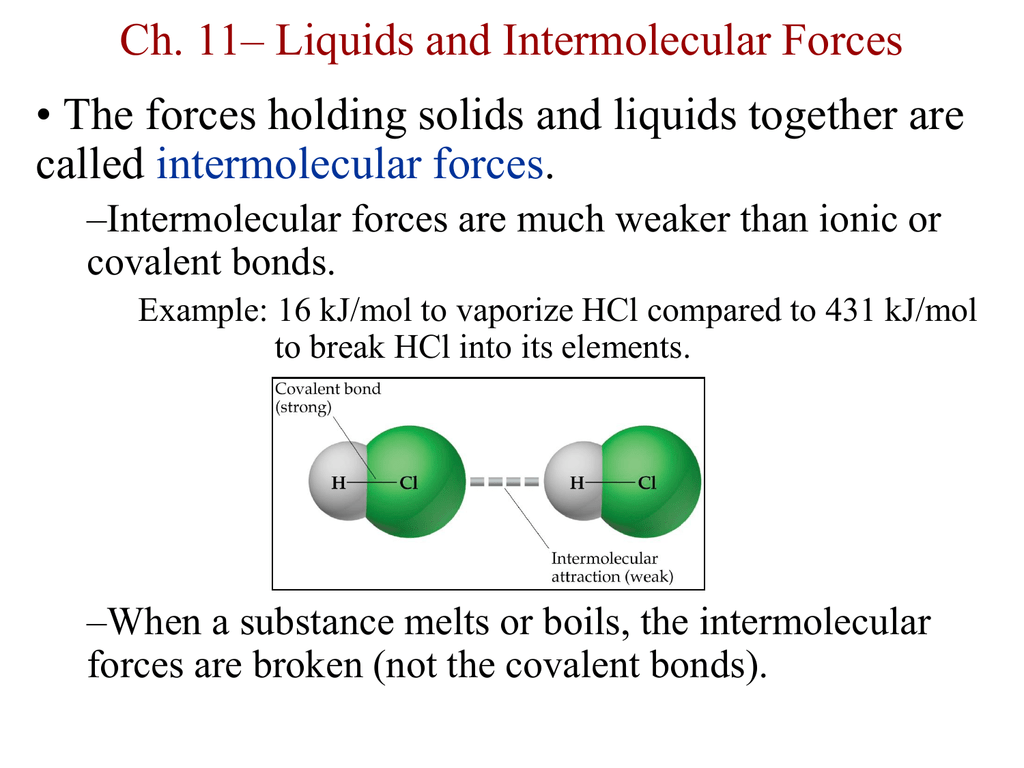
Molecules are treated as point particles.


 0 kommentar(er)
0 kommentar(er)
